Abstract
Background: Next-generation sequencing (NGS) has led to the discovery of recurrently mutated genes in acute myeloid leukemia (AML). Molecular profiling in AML is important for prognostication and determining eligibility for targeted therapies. The use of NGS has increased in clinical practice, but variability in testing patterns still exist. The purpose of this study was to assess differences in the use of molecular genetic sequencing and use of targeted therapy for patients with AML based on area deprivation index (ADI) and insurance status.
Methods: 275 adult patients diagnosed with AML between 1/1/2010 and 9/1/2021 were included. Data were collected retrospectively, including patient demographics, clinical characteristics, cytogenetic and molecular data, and treatment. The ADI is a validated metric of neighborhood disadvantage that can be used to study geographically-based social determinants of health. Higher ADI values correlate to greater socioeconomic disadvantage. Patients were assigned an ADI rank between 1 and 100 based on zip code. Chi-squared, Fisher's exact test, and ANOVA were used to perform comparisons between groups. Survival analysis was performed using the Kaplan-Meier method.
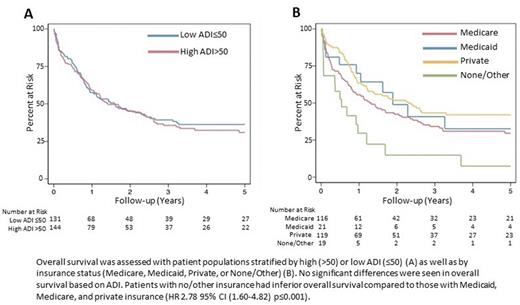
Results and Discussion: No significant differences were observed in baseline characteristics, disease characteristics, and treatment patterns based on ADI rank except for a higher prevalence of therapy-related AML in the low ADI group (15% vs. 6%, p=0.02). Genetic sequencing at diagnosis was also similar between ADI groups (82% vs. 87%, p=0.24). In contrast, Medicare insurance was associated with older age (p<0.001) and hypertension (p=0.002), while patients with Medicaid were more likely to be black (p=0.005). Patients with Medicaid or private insurance had genetic sequencing performed more often than those with Medicare or no/other insurance (91%, 91% vs. 78%, 79% respectively, p=0.03). Treatment patterns also varied by insurance status. Medicare and no/other insurance status was associated with fewer bone marrow transplants performed (36% and 11% respectively) compared to private (58%) and Medicaid (48%) insurance (p=0.001). Similarly, 9% of patients with Medicare and 0% of patients with no/other insurance received targeted therapy compared to 29% and 17% of patients with Medicaid or private insurance, respectively (p=0.01). ADI had no effect on overall survival (OS, HR=1.05, 95% CI 0.78-1.42, p=0.75). In contrast, no/other insurance was associated with inferior OS (HR=2.78, 95% CI 1.60-5.82, p<0.001), and a trend toward inferior OS was seen in patients with Medicare (HR 1.39, 95% CI 0.99-1.93, p=0.05). The presence of molecular data was associated with improved OS on univariate analysis (HR 0.36, 95% CI 0.25-0.51, p<0.001). In a subgroup analysis of patients diagnosed 2017-2021 when FLT3 inhibitors were clinically available, the presence of molecular data was associated with improved OS (HR 0.20 95% CI 0.11-0.37, p<0.001). FLT3 mutation was also associated with improved OS (HR 0.50, 95% CI 0.26-0.99, p=0.04). Receiving targeted therapy at any point did not influence OS in this subgroup (HR 0.53, 95% CI 0.22-1.27, p=0.16).
Conclusion: At our institution, there were no significant differences in practice patterns and patient outcomes based on ADI rank. Conversely, patients with Medicare or no/other insurance were less likely to have genetic sequencing performed, were treated with less intensive regimens, and had inferior OS. While insurance status may be directly contributing to these findings, these associations are likely multifactorial with other factors, such as age and comorbid conditions, also influencing treatment options and transplant eligibility in this patient population. Patients with Medicaid had equivalent usage of NGS and OS compared to patients with private insurance. This suggests that outcomes of patients with Medicaid are favorable when able to access care at a comprehensive cancer center. Finally, the presence of molecular data was associated with improved OS, likely due to better risk stratification and identification of those eligible for targeted therapies. Overall, these data support universal coverage of NGS for patients with AML and suggests that neighborhood disadvantage does not influence outcome of patients with AML able to receive treatment at a comprehensive cancer center.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal